Background
Burkitt lymphoma (BL) is a rare but highly aggressive B cell neoplasm, with treatment success rates exceeding 80% in the United States (US) (Blood, PMC:3682339). Although there are different presentations of BL, the treatment and prognosis are quite similar among them (Blood, PMID:15265787). Trends in outcomes of BL have been previously reported, however, sub-analysis for minorities like Hispanics (HI), have not been widely studied. Understanding ethnic disparities in outcomes and patterns of care are crucial given the growth of HI in the US (APMIS, PMID:23607450). There is an unmet need in this field; therefore, this population-based study aims to help understand the impact of ethnicity in clinical outcomes for BL.
Material and Methods
A retrospective analysis of patients diagnosed with BL recorded in the Texas Cancer Registry was carried out. Inclusion criteria was histopathologic proven BL using the International Classification of Diseases for Oncology Third Edition (ICD-O-3) code list. Patients were divided in HI and non-Hispanics (NH) for comparison. Key demographic, socioeconomic, clinical, and survival outcome variables were reviewed. All statistical testing was determined using Fisher's Exact test, Pearson's Chi-square test, T-test or Wilcoxon test, as appropriate. Survival analysis was calculated in years from date of primary diagnosis to date of death or last date of follow up. Survival distributions were described with Kaplan-Meier curves and significance of variation in median survival with ethnicity was assessed with log rank testing. All statistical testing was two-sided with a significance level of 5%. The R language [R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria] was used throughout.
Results
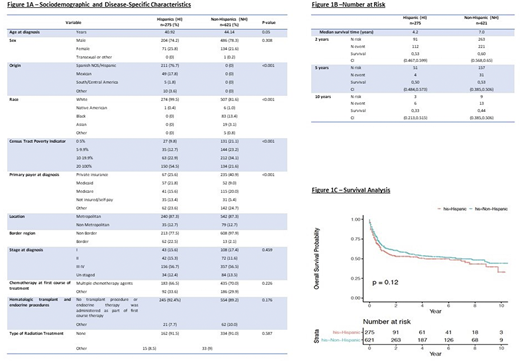
From 2006-2016, 896 patients (HI: n=275, NH: n=621) were diagnosed with BL. The median age at diagnosis for HI was 41 years vs 44 for NH [p-value 0.05]. Male sex predominated for HI and NH. Origin for HI was mainly Spain, Mexico and Central/South America. Both HI and NH were mainly identified as white; other races included black, asian and Native American [p-value <0.001]. For HI, the bracket of poverty indicator that prevailed was 20-100%; while majority of NH were in 10-19.99% [p-value <0.001]. There was a statistical significance regarding the primary payer at diagnosis [p-value <0.001]. HI had a similar distribution among private insurance (PI), Medicaid, Medicare and not insured/self-pay; while NH had primarily PI. The greatest proportion of HI and NH where located in the metropolitan, non-border area.
The majority of HI and NH were diagnosed at stage III-IV [p-value 0.459]. Treatment at diagnosis showed a similar pattern for HI and NH, choosing mainly chemotherapy. For both groups, most of the patients did not undergo transplant or radiation (Figure1A).
The median survival time for HI was 4.2 years and for NH was 7.0 years. Survival probability at 2, 5 and 10 years for HI was 0.53 (CI=0.467,0.599), 0.50 (CI=0.484,0.573) and 0.33 (CI=0.213,0.515), respectively. For NH it was 0.60 (CI=0.568,0.65), 0.53 (CI=0.385,0.506) and 0.44 (0.385,0.506), respectively (Figure1B). The overall survival probability at 10 years did not show a statistically significant difference for HI vs NH [p-value 0.12] (Figure1C).
Conclusions
Survival analysis at 10 years shows similar outcomes for HI vs NH; however, significant differences were observed in demographics and sociocultural variables. HI were diagnosed at a younger age, predominantly of Spanish origin and white race. More HI were uninsured compared to NH, as well as their poverty index was statistically higher. The fact that no variation was reported in overall survival may be explained by the use of standardized treatment. From our analysis, racial or economical variations do not seem to affect oncological outcomes in BL for HI in the US.
Diaz Duque:ADCT Therapeutics: Research Funding; Molecular Templates: Research Funding; AstraZeneca: Research Funding; Hutchinson Pharmaceuticals: Research Funding; Seattle Genetics: Speakers Bureau; Verastem: Speakers Bureau; AbbVie: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal